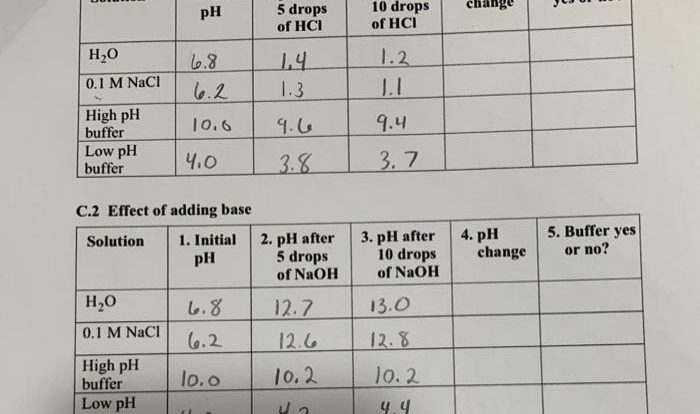
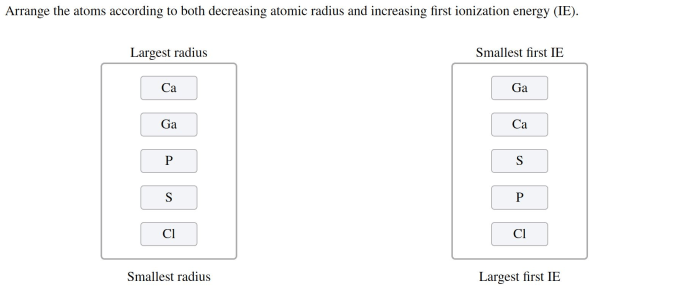
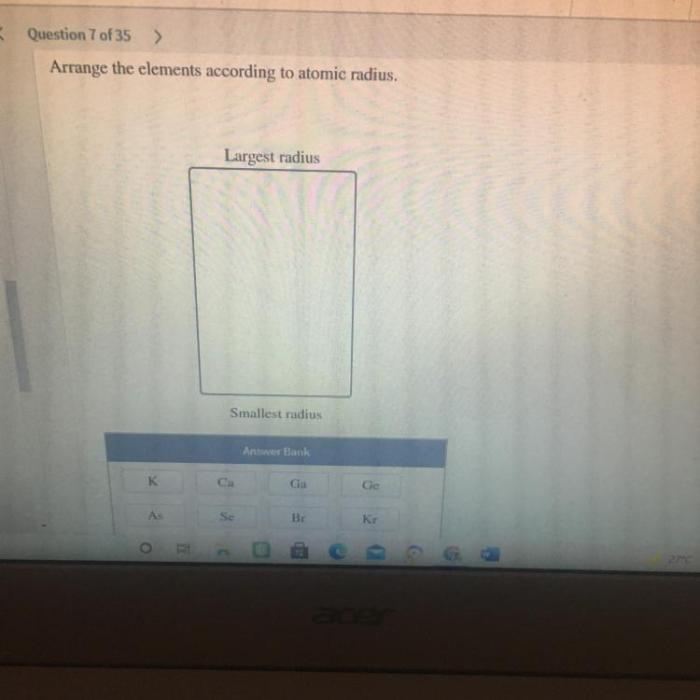
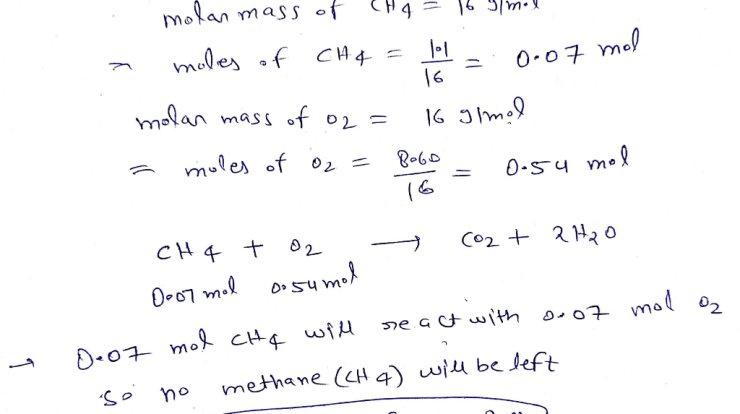Arrange the elements according to atomic radius. – Atomic radii, a fundamental property of elements, play a pivotal role in shaping their chemical behavior and physical properties. Arrange the elements according to atomic radius, and a fascinating tapestry of periodic trends emerges, offering insights into the intricate workings of the atomic world.
Delving into the factors that influence atomic radii, we uncover the interplay between electron shells, nuclear charge, and the periodic table’s organization. This understanding provides a foundation for exploring the profound impact of atomic radii on chemical reactivity, bond formation, and the stability of compounds.
Periodic Trends: Atomic Radius: Arrange The Elements According To Atomic Radius.
Atomic radius is a measure of the size of an atom, specifically the distance from the nucleus to the outermost electron shell. It is a crucial property that influences many chemical and physical properties of elements.
Generally, atomic radius decreases from left to right across a period and increases from top to bottom within a group. This is because the number of protons in the nucleus increases across a period, leading to a stronger attraction between the nucleus and the electrons, resulting in a smaller atomic radius.
Down a group, the number of electron shells increases, which increases the distance between the nucleus and the outermost electrons, leading to a larger atomic radius.
| Element | Atomic Number | Atomic Radius (pm) |
|---|---|---|
| Hydrogen | 1 | 53 |
| Helium | 2 | 31 |
| Lithium | 3 | 155 |
| Beryllium | 4 | 111 |
| Boron | 5 | 85 |
Factors that influence atomic radius include:
- Number of electron shells: As the number of electron shells increases, the atomic radius increases.
- Effective nuclear charge: The effective nuclear charge is the net positive charge experienced by the electrons. A higher effective nuclear charge leads to a smaller atomic radius.
Atomic Radius and Chemical Properties
Atomic radius plays a significant role in determining the chemical properties of elements:
- Reactivity: Elements with larger atomic radii are generally more reactive because their outermost electrons are farther from the nucleus and thus less tightly held.
- Ionization energy: Ionization energy is the energy required to remove an electron from an atom. Elements with larger atomic radii have lower ionization energies because the outermost electrons are farther from the nucleus and easier to remove.
- Electronegativity: Electronegativity is the ability of an atom to attract electrons. Elements with smaller atomic radii have higher electronegativities because their outermost electrons are closer to the nucleus and more strongly attracted to it.
Atomic radius also influences the formation of chemical bonds and the stability of compounds:
- Bond length: The bond length between two atoms is influenced by their atomic radii. Generally, atoms with larger atomic radii form longer bonds.
- Bond strength: The strength of a chemical bond is influenced by the atomic radii of the bonded atoms. Bonds between atoms with similar atomic radii tend to be stronger than bonds between atoms with significantly different atomic radii.
Ionic radius is the radius of an ion, which is formed when an atom gains or loses electrons. Ionic radius is typically larger than atomic radius because the loss of electrons reduces the effective nuclear charge, leading to a larger radius.
Applications of Atomic Radius
The knowledge of atomic radius is crucial in various fields:
- Materials science: Atomic radius is used to design and engineer materials with specific properties, such as strength, hardness, and conductivity.
- Catalysis: Atomic radius plays a role in understanding the catalytic activity of materials, as the size and shape of the catalyst’s active sites can influence its ability to interact with reactants.
- Nanotechnology: Atomic radius is important in the design and synthesis of nanomaterials, where the precise control of atomic arrangements is essential for achieving desired properties.
In biological systems, atomic radius influences the behavior of atoms and molecules:
- Protein structure: The atomic radii of amino acids determine the folding and stability of proteins.
- Enzyme catalysis: The active sites of enzymes are designed to accommodate specific molecules, and the atomic radii of the enzyme’s amino acids play a role in determining the enzyme’s specificity and catalytic efficiency.
Experimental Determination of Atomic Radius
Atomic radius can be determined experimentally using various techniques:
- X-ray crystallography: This technique involves analyzing the diffraction of X-rays by a crystal lattice to determine the atomic positions and calculate the atomic radii.
- Electron microscopy: This technique uses a beam of electrons to image atoms and molecules, allowing for the direct measurement of atomic radii.
These techniques have challenges and limitations, such as the need for high-quality crystals or the potential for beam damage in electron microscopy. Despite these challenges, experimental data has played a crucial role in refining our understanding of atomic radii.
Expert Answers
What factors influence atomic radii?
Atomic radii are influenced by the number of electron shells, the effective nuclear charge, and the shielding effect of inner electrons.
How does atomic radius affect chemical reactivity?
Smaller atomic radii generally lead to higher chemical reactivity due to increased electron density and stronger electrostatic interactions.
What is the relationship between atomic radius and electronegativity?
Electronegativity tends to increase across a period and decrease down a group in the periodic table, inversely correlating with atomic radius.