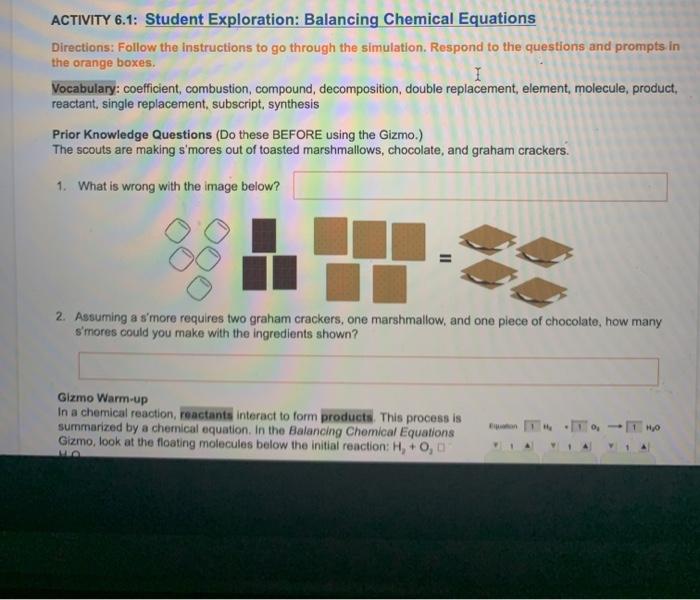
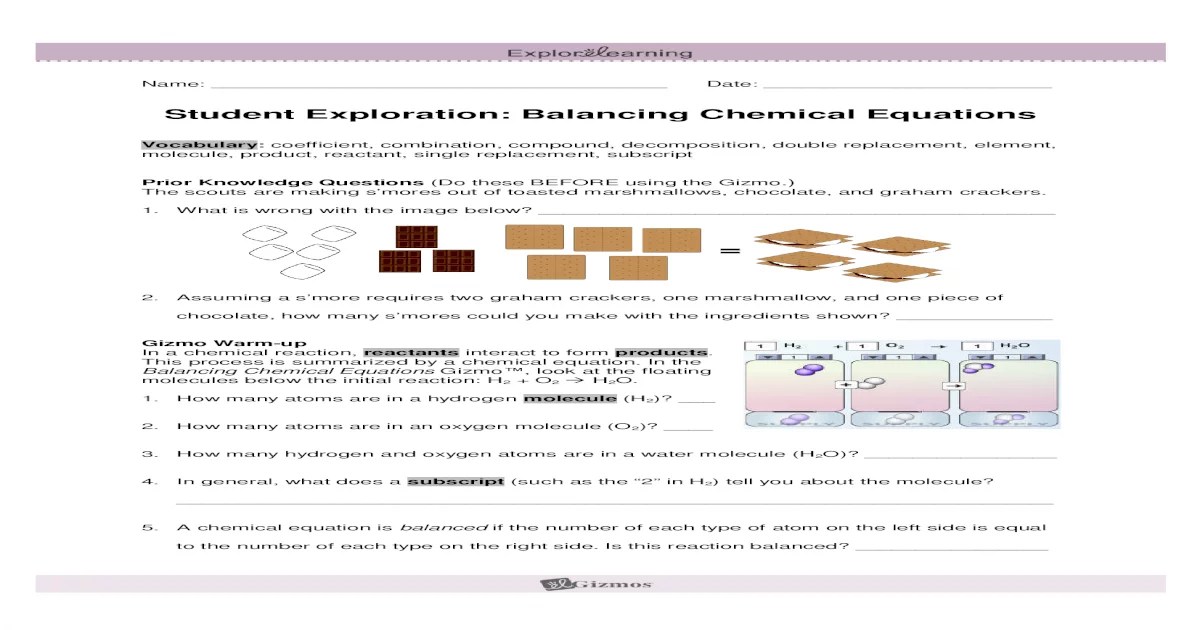
Student exploration balancing chemical equations opens the door to a captivating educational journey, where learners actively engage in the intricate world of chemical reactions and stoichiometry. By delving into the intricacies of balancing equations, students not only acquire a deeper understanding of chemical principles but also develop critical thinking and problem-solving skills.
Through hands-on activities, interactive simulations, and real-life experiments, this exploration empowers students to grasp the significance of balancing equations in predicting reaction outcomes and comprehending the quantitative relationships between reactants and products. This article provides a comprehensive framework for educators to guide students in their exploration of balancing chemical equations, fostering a deeper appreciation for the elegance and precision of chemistry.
Student Exploration of Balancing Chemical Equations
Student exploration is a crucial aspect of learning, fostering deep understanding and critical thinking. Balancing chemical equations is a fundamental skill in chemistry, essential for comprehending chemical reactions and predicting their outcomes. This article provides a framework for student exploration of balancing chemical equations, enabling them to develop a strong foundation in this essential concept.
Methods for Student Exploration
Students can balance chemical equations through a step-by-step process:
- Identify the reactants and products in the unbalanced equation.
- Count the atoms of each element on both sides of the equation.
- Adjust the coefficients (numbers in front of each chemical formula) to make the number of atoms of each element equal on both sides.
- Check the equation again to ensure it is balanced.
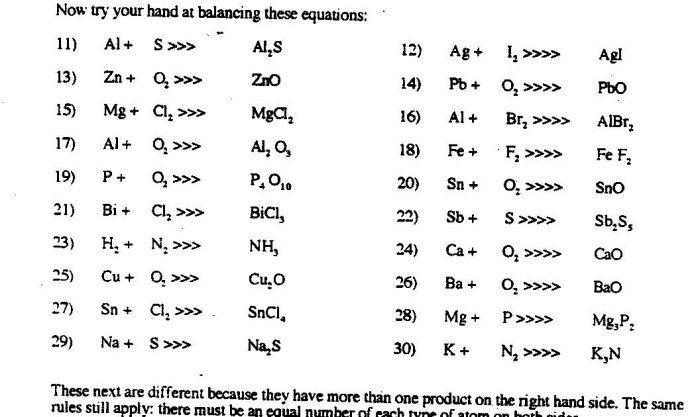
Different strategies for balancing equations include the half-reaction method and the oxidation number method. Students can explore these methods through practice and examples, such as:
CH4+ 2O 2→ CO 2+ 2H 2O
Tools and Resources
Online simulations and interactive tools can enhance student exploration of balancing chemical equations:
- Balancing Chemical Equations Simulator: https://phet.colorado.edu/sims/html/balancing-chemical-equations/latest/balancing-chemical-equations_en.html
- Chemical Equation Balancer: https://www.symbolab.com/solver/chemistry/chemical-equation-balancer
Websites, videos, and other resources provide additional support:
- Balancing Chemical Equations: https://www.khanacademy.org/science/chemistry/chemical-reactions/balancing-chemical-equations/v/how-to-balance-chemical-equations
- Balancing Chemical Equations Video Tutorial: https://www.youtube.com/watch?v=7FoEx_k606Q
Activities and Experiments
Hands-on activities allow students to apply their knowledge of balancing chemical equations:
- Balancing Act:Students create their own unbalanced chemical equations and work in groups to balance them.
- Reaction Lab:Students conduct experiments that demonstrate the importance of balancing equations, such as the reaction between sodium bicarbonate and vinegar.
Safety guidelines must be followed during all activities and experiments.
Assessment and Evaluation: Student Exploration Balancing Chemical Equations
Assessment of student understanding of balancing chemical equations can be done through:
- Formative Assessments:Quizzes, worksheets, and class discussions.
- Summative Assessments:Lab reports, exams, and projects.
Feedback is crucial for student growth and should be provided throughout the assessment process.
Expert Answers
What is the significance of balancing chemical equations?
Balancing chemical equations is crucial for understanding the stoichiometry of reactions, predicting reaction outcomes, and determining the quantitative relationships between reactants and products.
How can student exploration enhance the learning of balancing chemical equations?
Student exploration provides a hands-on and engaging approach that allows students to actively participate in the learning process, develop critical thinking skills, and gain a deeper understanding of the concepts.
What are some effective methods for student exploration of balancing chemical equations?
Effective methods include step-by-step instructions, interactive simulations, hands-on activities, real-life experiments, and group discussions.
How can technology be integrated into student exploration of balancing chemical equations?
Technology offers a range of interactive simulations, online resources, and visualization tools that can enhance student engagement and understanding.