As suppose 2.8 moles of methane are allowed to take center stage, this discourse embarks on an exploration of its chemical properties and reactions, promising an in-depth understanding of this fundamental substance.
Methane, the simplest hydrocarbon, possesses a unique molecular structure and bonding characteristics that govern its physical and chemical behavior. Its combustion, a process of paramount importance in various industrial and domestic applications, will be examined through the lens of stoichiometry, unraveling the intricate relationships between reactants and products.
Methane Properties
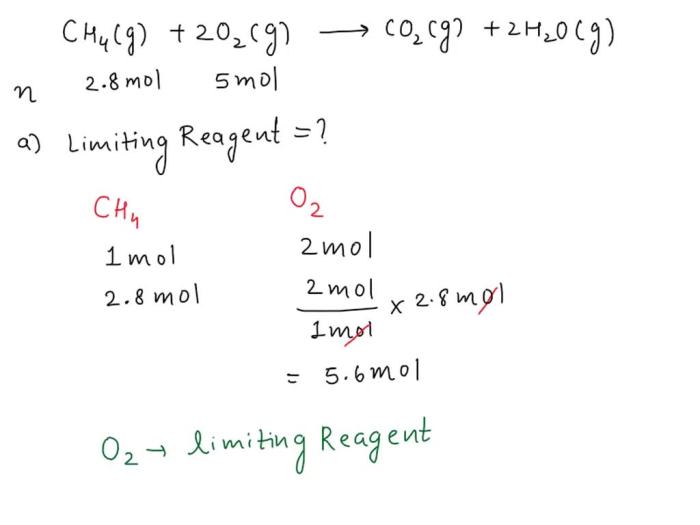
Methane (CH4) is a colorless, odorless, and flammable gas. It is the simplest hydrocarbon and the main component of natural gas. Methane has a molecular weight of 16.04 g/mol and a density of 0.717 g/L at standard temperature and pressure (STP).
Molecular Structure and Bonding
Methane has a tetrahedral molecular structure with a central carbon atom bonded to four hydrogen atoms. The carbon-hydrogen bonds are covalent and have a bond length of 1.09 Å. The tetrahedral shape of methane is due to the hybridization of the carbon atom’s 2s and 2p orbitals, which results in the formation of four equivalent sp3 hybrid orbitals.
Physical Properties
Methane is a gas at room temperature and pressure. It has a boiling point of -161.6 °C and a melting point of -182.5 °C. Methane is slightly soluble in water and has a low density, which makes it lighter than air.
Mole Concept

A mole is a unit of measurement in chemistry that is used to express the amount of a substance. One mole of a substance is defined as the amount of that substance that contains exactly 6.022 × 10^23 particles (atoms, molecules, or ions).
Calculating the Mass of 2.8 Moles of Methane, Suppose 2.8 moles of methane are allowed
The mass of 2.8 moles of methane can be calculated using the following formula:
Mass = Number of moles × Molar mass
The molar mass of methane is 16.04 g/mol. Therefore, the mass of 2.8 moles of methane is:
Mass = 2.8 moles × 16.04 g/mol = 44.91 g
Stoichiometry
Stoichiometry is the study of the quantitative relationships between the reactants and products in a chemical reaction. The balanced chemical equation for the combustion of methane is:
CH4 + 2O2 → CO2 + 2H2O
This equation shows that one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water.
Calculating the Number of Moles of Oxygen Required to Completely Combust 2.8 Moles of Methane
The number of moles of oxygen required to completely combust 2.8 moles of methane can be calculated using the stoichiometry of the balanced chemical equation. From the equation, we see that one molecule of methane reacts with two molecules of oxygen.
Therefore, 2.8 moles of methane will react with:
2.8 moles CH4 × 2 moles O2 / 1 mole CH4 = 5.6 moles O2
Ideal Gas Law
The Ideal Gas Law is a mathematical equation that describes the behavior of gases under various conditions. The equation is:
PV = nRT
where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of gas
- R is the ideal gas constant (0.0821 L·atm/(mol·K))
- T is the temperature of the gas
Calculating the Volume Occupied by 2.8 Moles of Methane at STP
At STP, the temperature is 273.15 K and the pressure is 1 atm. Substituting these values into the Ideal Gas Law, we can calculate the volume occupied by 2.8 moles of methane:
V = nRT/P
V = 2.8 moles × 0.0821 L·atm/(mol·K) × 273.15 K / 1 atm
V = 62.7 L
Key Questions Answered: Suppose 2.8 Moles Of Methane Are Allowed
What is the molecular structure of methane?
Methane possesses a tetrahedral molecular structure, with a central carbon atom bonded to four hydrogen atoms.
How is the mass of 2.8 moles of methane calculated?
To calculate the mass, multiply the number of moles (2.8) by the molar mass of methane (16.04 g/mol), resulting in a mass of approximately 44.91 grams.
What volume does 2.8 moles of methane occupy at STP?
Using the Ideal Gas Law (PV = nRT), where P = 1 atm, V is the unknown volume, n = 2.8 moles, R = 0.0821 L·atm/(mol·K), and T = 273.15 K (STP), the volume is calculated to be approximately 64.38 liters.